NIST Request for Comments on March-in Rights: “Mad Libs” Dominate Individual Submissions While Opposition Dominates Organizational Comments
Background
The following summary is provided by the National Institute of Standards and Technology on regulatons.gov:
The National Institute of Standards and Technology (NIST) seeks comments on the Draft Interagency Guidance Framework for Considering the Exercise of March-In Rights, which reviews the factors that an agency may consider when deciding whether to exercise march-in rights. NIST requests information from the public on the proposed version of this guidance document to ensure that it is clear, and its application will both fulfill the purpose of march-in rights and uphold the policy and objectives of the Bayh-Dole Act. The information received in response to this RFI will inform NIST and the Interagency Working Group for Bayh-Dole (IAWGBD) in developing a final framework document that may be used by an agency when making a march-in decision. NIST will hold at least one informational webinar explaining the Draft Interagency Guidance Framework for Considering the Exercise of March-In Rights and how the public can submit comments. Details about the informational webinar(s), including dates, times and any registration requirements, will be announced at https://www.nist.gov/tpo/policy-coordination/bayh-dole-act.
Overall Comment Analysis:
The NIST Request for Information Regarding the Draft Interagency Guidance Framework for Considering the Exercise of March-In Rights (NIST-2023-0008-0001) drew significant attention from industry organizations and individual comments. RDVM Analytics performed an analysis of the sentiment reflected in the 51,847 non-duplicate comments available on regulations.gov. This analysis provided fascinating insights into the positions of various research organizations, advocacy groups, grassroots movements, and the use of template comments to bolster support for their viewpoints.
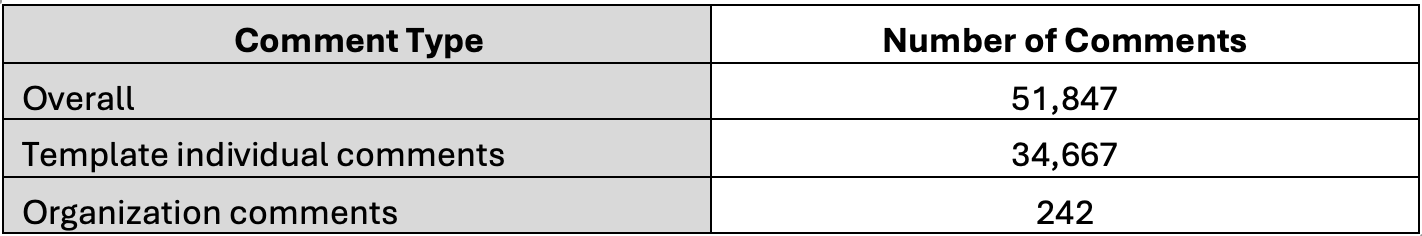
The table above showcases the breakdown of the comments submitted at the time of our analysis.
Organization Sentiment:
More than 200 organizations, spanning academia, patient and consumer advocacy, research organizations, labor, business, and life science trade groups weighed in overwhelmingly against the proposed policy change on march-in rights. Of the 242 organizations that submitted comments, only 9 supported the expansion of march-in rights. In our full report, RDVM Analytics analyzes their sentiment and summarizes their comment on the regulation.
Template Comments:
Template comments made up more than two-thirds of the comments submitted to the NIST. The majority of these comments were in support of the expansion of march-in rights, but several grassroots efforts spurred comments in opposition from various individuals.
To identify templated comments, RDVM Analytics groups any comments with over a 90% similarity together to encompass any diction changing or "Mad Libs" templates that are submitted to attempt to bypass similarity analysis.
Samples of both supporting and opposing templated comments are provided below.
Template Comment #1 (26,594 total): Supports
I support the use of march-in authority to lower prescription drug prices. Americans shouldn’t pay the highest prices in the world for medicines that were developed with our tax dollars. The final guidelines must also ensure that the vast majority of drugs, which are already priced at egregious levels, will be included as candidates for use of march-in rights. We need these guidelines to be iron-clad and bring down the exorbitant prices of prescription drugs! Why do Americans have to pay more for the drugs they need and other countries don't? Of the organizational comments submitted, all but 9 we're opposed to the expansion of march-in rights proposed by the NIST. Trade organizations, industry participants, and universities we're strongly opposed to the expansion of march-in rights. This showcases a strong opposition from industry interests towards this proposed legislation.
Template Comment #2 (3,825 total): Supports
The federal government should use its authority to lower prescription drug prices. It’s not right that Americans often have to pay many times more than people in other countries for the same drugs — especially when the drugs rely on government or taxpayer-funded research. Please update this guidance to direct agencies to use their march-in rights to license competition when Americans are being charged more for a medication than people in other high-income countries so we get a fair deal for prescription drugs our tax dollars paid to invent.
Template Comment #3 (3,241 total): Opposes
Maryland is a national leader in biopharmaceutical innovation, leading the country in the development of new treatments to treat many chronic and debilitating conditions. Our ability to develop and bring new medications to market is due, in part, to the incentivization of public and private partnerships and the protection of strong intellectual property (IP) rights. Our current IP system is the foundation for new treatments and cures. However, the Biden administration's recently released guidelines around the use of march-in rights will decimate our local innovation ecosystem in Maryland as well as our national ability to develop new medications, respond to public health threats, and excel in biomedical innovation. March-in rights are intended to only be used in four specific circumstances and the National Institutes of Health (NIH) has historically denied every march-in request because the correct circumstances have never been met. Misusing march-in rights will have little to no impact on the cost of medicines, and will only hold back critical research and development for treatments that patients are waiting for. Please protect our nation's ability to bring new treatments and cures from a research lab to a patient's medicine cabinet. Weakening IP rights is a misguided attempt to lower health care costs for patients. Take action to protect patients, public and private partnerships, and our nation's leading life sciences industry.
Template Comment #4 (377 total): Supports
I support the use of “march-in” authority to lower prescription drug prices. Under the 1980 Bayh Dole Act, the U.S. Government retains “residual rights” to inventions developed using Federal dollars. These rights include the one called “march-in,” which grants licenses to third parties if the benefits of the invention are not available to the public on reasonable terms. The Administration sees “march-in rights” as an effective tool to reduce prescription drug costs. White House advisor Neera Tanden has said, “When Americans and the federal government invest in a drug, that brand has to be accessible to the public … if you can’t afford it, it’s not accessible.” Please use “march-in” authority to decrease prescription drug costs for Americans.
Template Comment #5 (304 total): Opposes
The proposed changes to the Bayh-Dole Act endanger the lifeline of small businesses and startups, especially in high-tech fields that depend on cutting-edge university research. Patents should be secure property rights, not be subject to government seizure. I urge you to withdraw this guidance.
Template Comment #6 (183 total): Opposes
I am writing today to ask that you not move forward with finalizing the recent draft framework. That framework suggests new ways that federal agencies could "march-in" and seize patent rights, deterring investment in any technology that results from federally-funded research. As someone who has a personal stake in the development of new medicines, I have grave concerns that if the government were to move forward, it would result in damage to public-private research collaboration and stifle the work scientists and researchers are doing to save lives. The government should be encouraging, not punishing, partnerships between the public and private sectors to benefit me and my family. I hope you recognize this and will not finalize the newly proposed march-in framework.
Template Comment #7 (143 total): Opposes
The proposed framework by NIST, involving pricing considerations in the exercise of march-in rights, severely compromises the integrity of federally funded research. It disregards the original intent of the Bayh-Dole Act, which was to encourage efficient commercialization of research without government interference in pricing. Such changes could hinder innovation and devalue federal research investments. Moreover, given the enormous scale of federal grantmaking and contracting, it potentially undermines patent rights throughout the economy.I urge you to withdraw this guidance. It's critically necessary for sustaining and growing an economy that creates job opportunities rather than gut entrepreneurially driven capitalism and free markets not to mention allowing for democracy and liberty to prevail!
Our Reports:
RDVM Analytics provides reports on sentiment within public commentary on past and upcoming regulatory proposals. Our customizable analysis reports provide interested stakeholders valuable information on prevailing sentiment on proposed regulations. Our full NIST-2023-0008-0001 report is available upon request.
